|
Diindolylmethane (DIM) Information Resource
Center
An
Initiative of Faculty Members and Research Fellows at the
University of California at Berkeley



Home
/ Formation /
Molecular Biology /
Clinical Applications /
Research at Cal / References
Overview of Diindolylmethane (DIM) Formation
Indole-3-carbinol
(I3C) is the immediate molecular precursor of Diindolylmethane (DIM).
Upon consumption of Brassica vegetables, the enzyme myrosinase is released from
the plants, which cleaves glucobrassicin to release I3C.
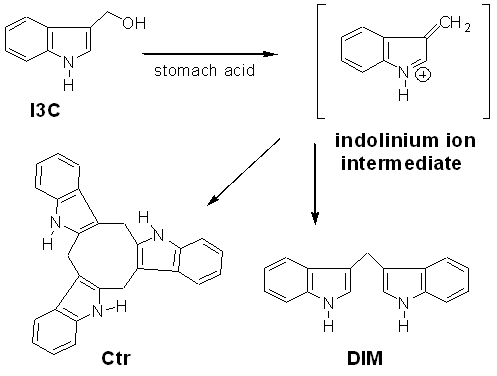
Structures of I3C and Two Acid Reaction Products.
I3C has been shown
to degrade fairly readily in aqueous systems to a reactive indolinium ion
intermediate. In the environment of the stomach, the indolinium ion reacts with
I3C or other indolinium ions to form oligomers such as Diindolylmethane (DIM) and
the Cyclic Trimer (Ctr).
In cellular environments, the indolinium ion binds non-specifically with free
thiols on proteins and glutathione.
Due to I3C's high
degree of reactivity and instability within the body, DIM is
more recommended as a dietary supplement than I3C as all of I3C's derivatives and their
variety of biological activities have not been fully explored. DIM, on the other
hand, is more stable and less reactive as a compound, with extensive human
clinical studies on its use as a dietary supplement.


© 2007-2021
Diindolylmethane Information Resource Center
|